 Company’s first product to begin shipping to wholesale partners in January.
Company’s first product to begin shipping to wholesale partners in January.
SCHAUMBURG, Ill.–(BUSINESS WIRE)–Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Melphalan Hydrochloride for Injection in the United States as a therapeutic equivalent generic for Alkeran® for Injection (melphalan hydrochloride) approved by the U.S. Food and Drug Administration. Melphalan Hydrochloride for Injection is indicated for the palliative treatment of patients with multiple myeloma for whom oral therapy is not appropriate.
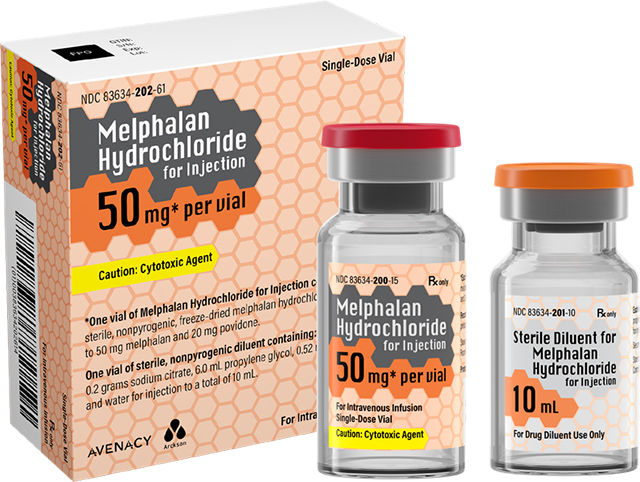
Avenacy’s Melphalan Hydrochloride for Injection is available in a kit containing a 50mg vial of lyophilized active ingredient and a 10mL vial of sterile diluent for admixture. This product is formulated to allow storage at room temperature, enabling wider use in medical settings through greater transportability and ease of access. In line with Avenacy’s mission to champion patient safety and streamline patient care, Melphalan Hydrochloride for Injection will feature the Company’s highly differentiated packaging and labeling to support accurate medication selection.
“Avenacy’s first product to market comes less than three months after our launch as a new specialty pharmaceutical company and is a testament to our seasoned team and strong relationships with partners. We are building on our momentum as we enter 2024 and plan to introduce several additional new products in the coming months,” said Jeff Yordon, Co-Founder and CEO of Avenacy. “Melphalan Hydrochloride for Injection will feature our proprietary product packaging and labeling, supporting our commitment to championing reliability, safety, and convenience for our customers. As Avenacy continues to grow, I look forward to working with our global partners to deliver more critical injectable medications to hospitals and healthcare providers.”
Avenacy is initiating commercial operations throughout the U.S. and will begin shipping Melphalan Hydrochloride for Injection to wholesale partners this week. The Company is supported by a global network of development and manufacturing partners, including Arthur Group as the Abbreviated New Drug Application (ANDA) holder and Qilu Pharmaceutical, Ltd. as the FDA-approved cGMP-certified contract manufacturing organization.
Melphalan Hydrochloride for Injection had U.S. sales of approximately $25 million for the twelve months ending in June 2023.1
Warning
Melphalan should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Severe bone marrow suppression with resulting infection or bleeding may occur.
Controlled trials comparing intravenous (IV) to oral melphalan have shown more myelosuppression with the IV formulation. Hypersensitivity reactions, including anaphylaxis, have occurred in approximately 2% of patients who received the IV formulation. Melphalan is leukemogenic in humans. Melphalan produces chromosomal aberrations in vitro and in vivo and, therefore, should be considered potentially mutagenic in humans.
Please see link for Full Prescribing Information including the Boxed Warning. Alkeran® is a registered trademark of Apotex, Inc.
1Source: IQVIA
About Avenacy
Avenacy is a U.S.-based specialty pharmaceutical company focused on supplying critical injectable medications used to treat patients in various medically supervised settings, from acute care hospitals to outpatient clinics and physician offices. Through a rigorous and optimized selection process, the Company is building out a pipeline of high-quality FDA approved injectable products in order to ensure a resilient portfolio that can meet the needs of today’s dynamic drug supply chain. With an experienced team, commitment to quality and reliability, and product offerings intended to facilitate safe and efficient patient care, Avenacy strives to be a trusted partner for essential medications.
Avenacy was launched in 2023 and is headquartered in Schaumburg, IL. For more information, please visit https://www.avenacy.com.
Media Contact
FTI Consulting Avenacy@fticonsulting.com